The menstrual cycle, under the control of the endocrine system, is necessary for reproduction. It may be divided into three distinct phases: menstruation, the follicular phase and the luteal phase.[2] Ovulation defines the transition from the follicular phase to the luteal phase. The length of each phase varies from woman to woman and cycle to cycle, though the average menstrual cycle is 28 days.[2] Hormonal contraception interferes with the normal hormonal changes with the aim of preventing reproduction.
Stimulated by gradually increasing amounts of estrogen in the follicular phase, menses slow then stop, and the lining of the uterus thickens. Follicles in the ovary begin developing under the influence of a complex interplay of hormones, and after several days one or occasionally two become dominant (non-dominant follicles atrophy and die). Approximately mid-cycle, 24-36 hours after the Luteinizing Hormone (LH) surges, the dominant follicle releases an ovum, or egg in an event called ovulation. After ovulation, the egg only lives for 24 hours or less without fertilization while the remains of the dominant follicle in the ovary become a corpus luteum; this body has a primary function of producing large amounts of progesterone. Under the influence of progesterone, the endometrium (uterine lining) changes to prepare for potential implantation of an embryo to establish a pregnancy. If implantation does not occur within approximately two weeks, the corpus luteum will involute, causing sharp drops in levels of both progesterone and estrogen. These hormone drops cause the uterus to shed its lining in a process termed menstruation.
In the menstrual cycle, changes occur in the female reproductive system as well as other systems (which lead to breast tenderness or mood changes, for example). A woman's first menstruation is termed menarche, and occurs typically around age 12. The end of a woman's reproductive phase is called the menopause, which commonly occurs somewhere between the ages of 45 and 55.
1 Terminology
2 Phases
2.1 Menstruation
2.2 Follicular phase
2.3 Ovulation
2.4 Luteal phase
3 Fertile window
4 Effect on other systems
4.1 Cycle abnormalities
5 Ovulation suppression
5.1 Hormonal contraception
5.2 Lactational amenorrhea
6 Etymological and biological associations
6.1 Nightlighting and the moon
6.2 Estrus and menstruation
7 References
Terminology
The menarche is one of the later stages of puberty in girls. The average age of menarche in humans is 12 years, but is normal anywhere between ages 8 and 16. Factors such as heredity, diet and overall health can accelerate or delay menarche.[3] The cessation of menstrual cycles at the end of a woman's reproductive period is termed menopause. The average age of menopause in women is 50 years, with anywhere between 40 and 58 being common. Menopause before age 35 is considered premature. The age of menopause is largely a result of genetics; however, illnesses, certain surgeries, or medical treatments may cause menopause to occur earlier.[4]The length of a woman's menstrual cycle will typically vary, with some shorter cycles and some longer cycles. A woman who experiences variations of less than eight days between her longest cycles and shortest cycles is considered to have regular menstrual cycles. It is unusual for a woman to experience cycle length variations of less than four days. Length variation between eight and 20 days is considered moderately irregular. Variation of 21 days or more between a woman's shortest and longest cycle lengths is considered very irregular (see cycle abnormalities).[5]
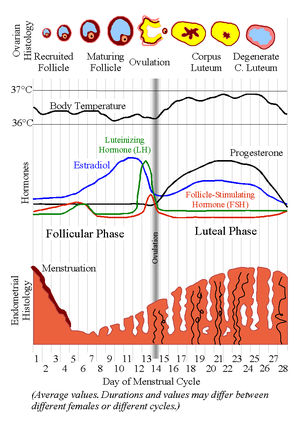
Phases
The menstrual cycle is divided into several phases. The average length of each phase is shown below:| Name of phase | Average start day assuming a 28-day cycle |
Average end day |
|---|---|---|
| menstrual phase | 1 | 4 |
| follicular phase (also known as proliferative phase) | 5 | 13 |
| ovulation (not a phase, but an event dividing phases) | 14 | 14 |
| luteal phase (also known as secretory phase) | 15 | 26 |
| ischemic phase (some sources group this with luteal phase) | 27 | 28 |
Menstruation
Menstruation is also called menstrual bleeding, menses, catamenia or a period. The flow of menses normally serves as a sign that a woman has not become pregnant. (However, this cannot be taken as certainty, as a number of factors can cause bleeding during pregnancy; some factors are specific to early pregnancy, and some can cause heavy flow.)[6][7][8] During the reproductive years, failure to menstruate may provide the first indication to a woman that she may have become pregnant.Eumenorrhea denotes normal, regular menstruation that lasts for a few days (usually 3 to 5 days, but anywhere from 2 to 7 days is considered normal).[9][10] The average blood loss during menstruation is 35 milliliters with 10–80 ml considered normal.[11] (Because of this blood loss, women are more susceptible to iron deficiency than men are.)[12] An enzyme called plasmin inhibits clotting in the menstrual fluid.[13] Cramping in the abdomen, back, or upper thighs is common during the first few days of menstruation. When menstruation begins, symptoms of premenstrual syndrome (PMS) such as breast tenderness and irritability generally decrease.[10] Many sanitary products are marketed to women for use during their menstruation.
Follicular phase
This phase is also called the proliferative phase because a hormone causes the lining of the uterus to grow, or proliferate, during this time.[2]Through the influence of a rise in follicle stimulating hormone (FSH) during the first days of the cycle, a few ovarian follicles are stimulated.[2] These follicles, which were present at birth[2] and have been developing for the better part of a year in a process known as folliculogenesis, compete with each other for dominance. Under the influence of several hormones, all but one of these follicles will stop growing, while one dominant follicle in the ovary will continue to maturity. The follicle that reaches maturity is called a tertiary, or Graafian, follicle, and it forms the ovum.[2]
As they mature, the follicles secrete increasing amounts of estradiol, an estrogen. The estrogens initiate the formation of a new layer of endometrium in the uterus, histologically identified as the proliferative endometrium. The estrogen also stimulates crypts in the cervix to produce fertile cervical mucus, which may be noticed by women practicing fertility awareness.[14]
Ovulation
An ovary about to release an egg.Numbered diagram with levels of estradiol, FSH and LH during the menstrual cycle.[15]
During the follicular phase, estradiol suppresses production of luteinizing hormone (LH) from the anterior pituitary gland. When the egg has nearly matured, levels of estradiol reach a threshold above which they stimulate production of LH. (These opposite responses of LH to estradiol may be enabled by the presence of two different estrogen receptors in the hypothalamus: estrogen receptor alpha—which is responsible for the negative feedback estradial-LH loop—and estrogen receptor beta—which is responsible for the positive estradiol-LH relationship.)[16] In the average cycle this LH surge starts around cycle day 12 and may last 48 hours.
The release of LH matures the egg and weakens the wall of the follicle in the ovary, causing the fully developed follicle to release its secondary oocyte.[2] The secondary oocyte promptly matures into an ootid and then becomes a mature ovum. The mature ovum has a diameter of about 0.2 mm.[17]
Which of the two ovaries—left or right—ovulates appears essentially random; no known left/right co-ordination exists.[18] Occasionally, both ovaries will release an egg;[18] if both eggs are fertilized, the result is fraternal twins.[19]
After being released from the ovary, the egg is swept into the fallopian tube by the fimbria, which is a fringe of tissue at the end of each fallopian tube. After about a day, an unfertilized egg will disintegrate or dissolve in the fallopian tube.[2]
Fertilization by a spermatozoon, when it occurs, usually takes place in the ampulla, the widest section of the fallopian tubes. A fertilized egg immediately begins the process of embryogenesis, or development. The developing embryo takes about three days to reach the uterus and another three days to implant into the endometrium.[2] It has usually reached the blastocyst stage at the time of implantation.
In some women, ovulation features a characteristic pain called mittelschmerz (German term meaning middle pain).[10] The sudden change in hormones at the time of ovulation sometimes also causes light mid-cycle blood flow.[20]
Luteal phase
The luteal phase is also called the secretory phase. An important role is played by the corpus luteum, the solid body formed in an ovary after the egg has been released from the ovary into the fallopian tube. This body continues to grow for some time after ovulation and produces significant amounts of hormones, particularly progesterone.[2] Progesterone plays a vital role in making the endometrium receptive to implantation of the blastocyst and supportive of the early pregnancy; it also has the side effect of raising the woman's basal body temperature.[21]After ovulation, the pituitary hormones FSH and LH cause the remaining parts of the dominant follicle to transform into the corpus luteum, which produces progesterone and estrogens. The hormones produced by the corpus luteum also suppress production of the FSH and LH that the corpus luteum needs to maintain itself. Consequently, the level of FSH and LH fall quickly over time, and the corpus luteum subsequently atrophies.[2] Falling levels of progesterone trigger menstruation and the beginning of the next cycle. From the time of ovulation until progesterone withdrawal has caused menstruation to begin, the process typically takes about two weeks, with ten to sixteen days considered normal. For an individual woman, the follicular phase often varies in length from cycle to cycle; by contrast, the length of her luteal phase will be fairly consistent from cycle to cycle.[22]
The loss of the corpus luteum can be prevented by fertilization of the egg; the resulting embryo produces human chorionic gonadotropin (hCG), which is very similar to LH and which can preserve the corpus luteum. Because the hormone is unique to the embryo, most pregnancy tests look for the presence of hCG.[2]
Fertile window
The most fertile period (the time with the highest likelihood of pregnancy resulting from sexual intercourse) covers the time from some 5 days before until 1–2 days after ovulation.[23] In an average 28 day cycle with a 14-day luteal phase, this corresponds to the second and the beginning of the third week. However, few cycles are exactly average. A variety of methods have been developed to help individual women estimate the relatively fertile and the relatively infertile days in the cycle: these systems are called fertility awareness.Fertility awareness methods that rely on cycle length records alone are called calendar-based methods.[24] Methods that require observation of one or more of the three primary fertility signs (basal body temperature, cervical mucus, and cervical position)[25] are known as symptoms-based methods.[24] Urine test kits are available that detect the LH surge that occurs 24 to 36 hours before ovulation; these are known as ovulation predictor kits (OPKs).[26] Computerized devices that interpret basal body temperatures, urinary test results, or changes in saliva are called fertility monitors.
A woman's fertility is also affected by her age.[27] As a woman's total egg supply is formed in fetal life,[28] to be ovulated decades later, it has been suggested that this long lifetime may make the chromatin of eggs more vulnerable to division problems, breakage, and mutation than the chromatin of sperm, which are produced continuously during a man's reproductive life.
Effect on other systems
Some women with neurological conditions experience increased activity of their conditions at about the same time during each menstrual cycle. Many women with epilepsy have more seizures in a pattern linked to the menstrual cycle; this is called "catamenial epilepsy".[29] Different patterns seem to exist (such as seizures coinciding with the time of menstruation, or coinciding with the time of ovulation), and the frequency with which they occur has not been firmly established. Using one particular definition, one group of scientists found that around one-third of women with intractable partial epilepsy have catamenial epilepsy.[29][30][31] An effect of hormones has been proposed, in which progesterone declines and estrogen increases would trigger seizures.[32] Studies by medical journals have found that women experiencing menses are 1.68 percent more likely to commit suicide.[33]Mice have been used as an experimental system to investigate possible mechanisms by which levels of sex steroid hormones might regulate nervous system function. During the part of the mouse estrous cycle when progesterone is highest, the level of nerve-cell GABA receptor subtype delta was high. Since these GABA receptors are inhibitory, nerve cells with more delta receptors are less likely to fire than cells with lower numbers of delta receptors. During the part of the mouse estrous cycle when estrogen levels are higher than progesterone levels, the number of delta receptors decrease, increasing nerve cell activity, in turn increasing anxiety and seizure susceptibility.[34]
Estrogen levels may affect thyroid behavior.[35] For example, during the luteal phase (when estrogen levels are lower), the velocity of blood flow in the thyroid is lower than during the follicular phase (when estrogen levels are higher).[36]
Among women living closely together, the onsets of menstruation may tend to synchronize somewhat. This McClintock effect was first described in 1971, and possibly explained by the action of pheromones in 1998.[37] However, subsequent research has called this hypothesis into question.[38]
Cycle abnormalities
Infrequent or irregular ovulation is called oligoovulation.[39] The absence of ovulation is called anovulation. Normal menstrual flow can occur without ovulation preceding it: an anovulatory cycle. In some cycles, follicular development may start but not be completed; nevertheless, estrogens will form and will stimulate the uterine lining. Anovulatory flow resulting from a very thick endometrium caused by prolonged, continued high estrogen levels is called estrogen breakthrough bleeding. Anovulatory bleeding triggered by a sudden drop in estrogen levels is called estrogen withdrawal bleeding.[40] Anovulatory cycles commonly occur prior to menopause (perimenopause) and in women with polycystic ovary syndrome.[41]Very little flow (less than 10ml) is called hypomenorrhea. Regular cycles with intervals of 21 days or fewer are polymenorrhea; frequent but irregular menstruation is known as metrorrhagia. Sudden heavy flows or amounts in excess of 80 ml are termed menorrhagia.[42] Heavy menstruation that occurs frequently and irregularly is menometrorrhagia. The term for cycles with intervals exceeding 35 days is oligomenorrhea.[43] Amenorrhea refers to more than three[42] to six[43] months without menses (in the absence of pregnancy) during a woman's reproductive years.
Ovulation suppression
Hormonal contraception
Half-used blister pack of a combined oral contraceptive. The white pills are placebos.While some forms of birth control do not affect the menstrual cycle, hormonal contraceptives work by disrupting it. Progestogen negative feedback decreases the pulse frequency of gonadotropin-releasing hormone (GnRH) release by the hypothalamus, which decreases the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) by the anterior pituitary. Decreased levels of FSH inhibit follicular development, preventing an increase in estradiol levels. Progestogen negative feedback and the lack of estrogen positive feedback on LH release prevent a mid-cycle LH surge. Inhibition of follicular development and the absence of a LH surge prevent ovulation.[44][45][46]
The degree of ovulation suppression in progestogen-only contraceptives depends on the progestogen activity and dose. Low dose progestogen-only contraceptives—traditional progestogen only pills, subdermal implants Norplant and Jadelle, and intrauterine system Mirena—inhibit ovulation in ~50% of cycles and rely mainly on other effects, such as thickening of cervical mucus, for their contraceptive effectiveness.[47] Intermediate dose progestogen-only contraceptives—the progestogen-only pill Cerazette and the subdermal implant Implanon—allow some follicular development but more consistently inhibit ovulation in 97–99% of cycles. The same cervical mucus changes occur as with very low dose progestogens. High dose progestogen-only contraceptives—the injectables Depo-Provera and Noristerat—completely inhibit follicular development and ovulation.[47]
Combined hormonal contraceptives include both an estrogen and a progestogen. Estrogen negative feedback on the anterior pituitary greatly decreases the release of FSH, which makes combined hormonal contraceptives more effective at inhibiting follicular development and preventing ovulation. Estrogen also reduces the incidence of irregular breakthrough bleeding.[44][45][46] Several combined hormonal contraceptives—the pill, NuvaRing, and the contraceptive patch—are usually used in a way that causes regular withdrawal bleeding. In a normal cycle, menstruation occurs when estrogen and progesterone levels drop rapidly.[21] Temporarily discontinuing use of combined hormonal contraceptives (a placebo week, not using patch or ring for a week) has a similar effect of causing the uterine lining to shed. If withdrawal bleeding is not desired, combined hormonal contraceptives may be taken continuously, although this increases the risk of breakthrough bleeding.
Lactational amenorrhea
Breastfeeding causes negative feedback to occur on pulse secretion of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH). Depending on the strength of the negative feedback, breastfeeding women may experience complete suppression of follicular development, follicular development but no ovulation, or normal menstrual cycles may resume.[48] Suppression of ovulation is more likely when suckling occurs more frequently.[5] The production of prolactin in response to suckling is important to maintaining lactational amenorrhea.[49] On average, women who are fully breastfeeding whose infants suckle frequently experience a return of menstruation at fourteen and a half months postpartum. There is a wide range of response between individual breastfeeding women, however, with some experiencing return of menstruation at two months and others remaining amenorrheic for up to 42 months postpartum.[50]Etymological and biological associations
Nightlighting and the moon
The word "menstruation" is etymologically related to "moon". The terms "menstruation" and "menses" are derived from the Latin mensis (month), which in turn relates to the Greek mene (moon) and to the roots of the English words month and moon—reflecting the fact that the moon also takes close to 28 days to revolve around the Earth (actually 27.32 days). The synodical lunar month, the period between two new moons (or full moons), is 29.53 days long.Some authors believe women in traditional societies without nightlighting ovulated with the full moon and menstruated with the new moon.[51] A few studies in both humans[52] and animals[53] have found that artificial light at night does influence the menstrual cycle in humans and the estrus cycle in mice (cycles are more regular in the absence of artificial light at night), though none have demonstrated the synchronization of women's menstrual cycles with the lunar cycle. It has also been suggested that bright light exposure in the morning promotes more regular cycles.[54] One author has suggested that sensitivity of women's cycles to nightlighting is caused by nutritional deficiencies of certain vitamins and minerals.[55]
Other animals' menstrual cycles may be greatly different from lunar cycles: while the average cycle length in orangutans is the same as in humans—28 days[56]—the average for chimpanzees is 35 days.[57] Some take this as evidence that the average length of humans' cycle is most likely a coincidence. [58] [59] [60] [61] [62]
Estrus and menstruation
Females of most mammal species advertise fertility to males with visual behavioral cues, pheromones, or both.[63] This period of advertised fertility is known as estrus or heat.[63] In species that experience estrus, females are generally only receptive to copulation while they are in heat[63] (dolphins are an exception).[64] In the estrous cycles of most placental mammals, if no fertilization takes place, the uterus reabsorbs the endometrium. This breakdown of the endometrium without vaginal discharge is sometimes called covert menstruation.[65] Overt menstruation (where there is blood flow from the vagina) occurs primarily in humans and close evolutionary relatives such as chimpanzees.[1] Some species, such as domestic dogs, experience small amounts of vaginal bleeding while in heat; this discharge has a different physiologic cause than menstruation.[66]A few mammals do not experience obvious, visible signs of fertility (concealed ovulation). In humans, while women can be taught to recognize their own level of fertility (fertility awareness), whether men can detect fertility in women is debated; recent studies have given conflicting results.[67][68] Orangutans also lack visible signs of impending ovulation.[56] Also, it has been said that the extended estrus period of the bonobo (reproductive-age females are in heat for 75% of their menstrual cycle)[69] has a similar effect to the lack of a "heat" in human females.[70]
References
1. Strassmann BI (1996). "The evolution of endometrial cycles and menstruation". Q Rev Biol 71 (2): 181–220. doi:10.1086/419369. PMID 8693059.2. Losos, Jonathan B.; Raven, Peter H.; Johnson, George B.; Singer, Susan R. (2002). Biology. New York: McGraw-Hill. pp. 1207–09. ISBN 0-07-303120-8.
3. "At what age does a girl get her first period?," from Menstruation and the Menstrual Cycle, National Women's Health Information Center (accessed June 11, 2005)
4. Shuman, Tracy (February 2006). "Your Guide to Menopause". WebMD. Retrieved 2006-12-16.
5. Kippley, John; Sheila Kippley (1996). The Art of Natural Family Planning (4th ed.). Cincinnati, OH: The Couple to Couple League. pp. 92. ISBN 0-926412-13-2.
6. Greenfield, Marjorie (2001-09-17). "Subchorionic Hematoma in Early Pregnancy". Ask Our Experts. DrSpock.com. Retrieved 2008-09-21.
7. Anderson-Berry, Ann L; Terence Zach (2007-12-10). "Vanishing Twin Syndrome". Emedicine.com (WebMD). Retrieved 2008-09-21.
8. Ko, Patrick; Young Yoon (2007-08-23). "Placenta Previa". Emedicine.com (WebMD). Retrieved 2008-09-21.
9. The National Women's Health Information Center (November 2002). "What is a typical menstrual period like?". U.S. Department of Health and Human Services. Retrieved 2005-06-11.
10. John M Goldenring (2007-02-01). "All About Menstruation". WebMD. Retrieved 2008-10-05.
11. David L Healy (2004-11-24). "Menorrhagia Heavy Periods - Current Issues". Monash University.
12. Harvey LJ, Armah CN, Dainty JR, et al. (October 2005). "Impact of menstrual blood loss and diet on iron deficiency among women in the UK". The British journal of nutrition 94 (4): 557–64. doi:10.1079/BJN20051493. PMID 16197581. Retrieved 2008-10-05.
13. Shiraishi M (August 1962). "Studies on identification of menstrual blood stain by fibrin-plate method. I. A study on the incoagulability of menstrual blood". Acta medicinae Okayama 16: 192–200. PMID 13977381. Retrieved 2008-10-05.
14. Weschler, Toni (2002). Taking Charge of Your Fertility (Revised ed.). New York: HarperCollins. pp. 359–361. ISBN 0-06-093764-5.
15. High-resolution reference ranges for estradiol, luteinizing hormone, and follicle-stimulating hormone in men and women using the AxSYM assay system Anand S. Dighea, Corresponding Author Contact Information, E-mail The Corresponding Author, Joseph M. Moya, Frances J. Hayesb and Patrick M. Slussa. doi:10.1016/j.clinbiochem.2004.10.011
16. Hu L, Gustofson RL, Feng H, et al. (October 2008). "Converse regulatory functions of estrogen receptor-alpha and -beta subtypes expressed in hypothalamic gonadotropin-releasing hormone neurons". Mol. Endocrinol. 22 (10): 2250–9. doi:10.1210/me.2008-0192. PMID 18701637.
17. Gray, Henry David (2000). "The Ovum". Anatomy of the human body. Philadelphia: Bartleby.com. ISBN 1-58734-102-6.
18. Ecochard R, Gougeon A (April 2000). "Side of ovulation and cycle characteristics in normally fertile women". Human reproduction (Oxford, England) 15 (4): 752–5. doi:10.1093/humrep/15.4.752. PMID 10739814.
19. "Multiple Pregnancy: Twins or More - Topic Overview". WebMD Medical Reference from Healthwise. 2007-07-24. Retrieved 2008-10-05.
20. Weschler (2002), p.65
21. Weschler (2002), pp.361-2
22. Weschler (2002), p.47
23. Weschler (2002), pp.242,374
24. Medical Eligibility Criteria for Contraceptive Use:Fertility awareness-based methods. Third edition. World Health Organization. 2004. Retrieved 2008-04-29.
25. Weschler (2002), p.52
26. MedlinePlus Encyclopedia LH urine test (home test)
27. Davis, Jeanie Lerche (2004-06-18). "Fertility Treatment Less Successful After 35". WebMD Health News. Retrieved 2008-09-21.
28. Krock, Lexi (October 2001). "Fertility Throughout Life". 18 Ways to Make a Baby. NOVA Online. Retrieved 2006-12-24. Haines, Cynthiac (January 2006). "Your Guide to the Female Reproductive System". The Cleveland Clinic Women's Health Center. WebMD. Retrieved 2006-12-24.
29. Herzog AG (March 2008). "Catamenial epilepsy: definition, prevalence pathophysiology and treatment". Seizure : the journal of the British Epilepsy Association 17 (2): 151–9. doi:10.1016/j.seizure.2007.11.014. PMID 18164632.
30. Herzog AG, Harden CL, Liporace J, et al. (September 2004). "Frequency of catamenial seizure exacerbation in women with localization-related epilepsy". Annals of neurology 56 (3): 431–4. doi:10.1002/ana.20214. PMID 15349872.
31. Herzog AG, Klein P, Ransil BJ (October 1997). "Three patterns of catamenial epilepsy". Epilepsia 38 (10): 1082–8. doi:10.1111/j.1528-1157.1997.tb01197.x. PMID 9579954.
32. Scharfman HE, MacLusky NJ (September 2006). "The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female". Epilepsia 47 (9): 1423–40. doi:10.1111/j.1528-1167.2006.00672.x. PMID 16981857. PMC 1924802.
33. Enrique Baca-García, Carmen Diaz-Sastre, Antonio Ceverino, Jeronimo Saiz-Ruiz, Francisco J. Diaz, and Jose de Leon (March/April 2003). "Association Between the Menses and Suicide Attempts: A Replication Study". Psychosomatic Medicine 65 (2): 237–44. doi:10.1097/01.PSY.0000058375.50240.F6. PMID 12651991. Retrieved 2008-12-02.
34. Maguire JL, Stell BM, Rafizadeh M, Mody I (June 2005). "Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety". Nat. Neurosci. 8 (6): 797–804. doi:10.1038/nn1469. PMID 15895085.
35. Doufas AG, Mastorakos G (2000). "The hypothalamic-pituitary-thyroid axis and the female reproductive system". Annals of the New York Academy of Sciences 900: 65–76. PMID 10818393.
36. Krejza J, Nowacka A, Szylak A, Bilello M, Melhem LY (July 2004). "Variability of thyroid blood flow Doppler parameters in healthy women". Ultrasound in medicine & biology 30 (7): 867–76. doi:10.1016/j.ultrasmedbio.2004.05.008. PMID 15313319.
37. Stern K, McClintock MK (1998). "Regulation of ovulation by human pheromones". Nature 392 (6672): 177–9. doi:10.1038/32408. PMID 9515961.
38. Adams, Cecil (2002-12-20). "Does menstrual synchrony really exist?". The Straight Dope. The Chicao Reader. Retrieved 2007-01-10.
39. Galan, Nicole (2008-04-16). "Oligoovulation". about.com. Retrieved 2008-10-12.
40. Weschler (2002), p.107
41. Anovulation at eMedicine
42. Menstruation Disorders at eMedicine
43. Oriel KA, Schrager S (October 1999). "Abnormal uterine bleeding". American Family Physician 60 (5): 1371–80; discussion 1381–2. PMID 10524483. Retrieved 2008-10-12.
44. Trussell, James (2007). "Contraceptive Efficacy". in Hatcher, Robert A., et al.. Contraceptive Technology (19th rev. ed.). New York: Ardent Media. ISBN 0-9664902-0-7.
45. Speroff, Leon; Darney, Philip D. (2005). "Oral Contraception". A Clinical Guide for Contraception (4th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 21–138. ISBN 0-781-76488-2.
46. Loose, Davis S.; Stancel, George M. (2006). "Estrogens and Progestins". in Brunton, Laurence L.; Lazo, John S.; Parker, Keith L. (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. pp. 1541–71. ISBN 0-07-142280-3.
47. Glasier, Anna (2006). "Contraception". in DeGroot, Leslie J.; Jameson, J. Larry (eds.). Endocrinology (5th ed.). Philadelphia: Elsevier Saunders. pp. 3000–1. ISBN 0721603769.
48. McNeilly AS (2001). "Lactational control of reproduction". Reprod. Fertil. Dev. 13 (7-8): 583–90. doi:10.1071/RD01056. PMID 11999309.
49. Stallings JF, Worthman CM, Panter-Brick C, Coates RJ (February 1996). "Prolactin response to suckling and maintenance of postpartum amenorrhea among intensively breastfeeding Nepali women". Endocr. Res. 22 (1): 1–28. PMID 8690004.
50. "Breastfeeding: Does It Really Space Babies?". The Couple to Couple League International. Internet Archive. 2008-01-17. Retrieved 2008-09-21., which cites: Sheila K. and John F. Kippley (November-December 1972). "The relation between breastfeeding and amenorrhea". Journal of obstetric, gynecologic, and neonatal nursing 1 (4): 15–21. PMID 4485271.
Sheila Kippley (November-December 1986 and January-February 1987). "Breastfeeding survey results similar to 1971 study". The CCL News 13 (3): 10. and 13(4): 5.
51. Cohen, Sari (February-March 2005). "Melatonin, menstruation, and the moon". Townsend Letter for Doctors and Patients. Retrieved 2008-09-21.
Knight, Chris; Camilla Power & Ian Watts (1995). "The Human Symbolic Revolution: A Darwinian Account" (PDF). Cambridge Archaeological Journal 5 (1): 75–114. doi:10.1017/S0959774300001190. Retrieved 2006-12-13.
Lacey, Louise (1975). Lunaception: a feminine odyssey into fertility and contraception. New York: Coward, McCann & Geoghegan. ISBN 0-698-10674-1.
52. Singer, Katie. "Fertility Awareness, Food, and Night-Lighting". Wise Traditions in Food, Farming and the Healing Arts, Spring 2004. See section on Night-Lighting.
53. Harder, Ben (Week of August 28, 2004). "Bright nights kindle cancers in mice". Science News 166 (9): 141.
54. Danilenko KV, Samoilova EA (2007). "Stimulatory effect of morning bright light on reproductive hormones and ovulation: results of a controlled crossover trial". PLoS clinical trials 2 (2): e7. doi:10.1371/journal.pctr.0020007. PMID 17290302.
55. Shannon, Marilyn M. (2001). Fertility, cycles & nutrition : how your diet affects your menstrual cycles & fertility (3rd ed.). Cincinnati, Ohio: Couple to Couple League International. pp. 71–2.
56. Knott, Cheryl (2003). "Orangutans: Reproduction and Life History". Gunung Palung Orangutan Project. Harvard University. Retrieved 2008-10-05.
57. Lacreuse A, Chennareddi L, Gould KG, et al. (September 2008). "Menstrual cycles continue into advanced old age in the common chimpanzee (Pan troglodytes)". Biology of reproduction 79 (3): 407–12. doi:10.1095/biolreprod.108.068494. PMID 18495682.
58. As cited by Adams, Cecil, "What's the link between the moon and menstruation?" (accessed 6 June 2006):
Abell, George O.; Barry Singer (1983). Science and the Paranormal: Probing the Existence of the Supernatural. Scribner Book Company. ISBN 0-684-17820-6.
59. Cutler WB (August 1980). "Lunar and menstrual phase locking". Am. J. Obstet. Gynecol. 137 (7): 834–9. PMID 7405975.
60. Friedmann E (June 1981). "Menstrual and lunar cycles". Am. J. Obstet. Gynecol. 140 (3): 350. PMID 7246643.
61. Law SP (1986). "The regulation of menstrual cycle and its relationship to the moon". Acta Obstet Gynecol Scand 65 (1): 45–8. PMID 3716780.
62. Zimecki M (2006). "The lunar cycle: effects on human and animal behavior and physiology". Postepy Hig Med Dosw (Online) 60: 1–7. PMID 16407788.
63. c "Estrus". Britannica Online. Retrieved 2008-10-05.
64. Mikkelson, Barbara and David P. (2007-06-29). "Buried Pleasure". Snopes.com. Retrieved 2008-10-05., which references: Diamond, Jared M. (1997). Why is sex fun?: the evolution of human sexuality. London: HarperCollins. ISBN 0-465-03127-7.
65. Profet M (1993). "Menstruation as a defense against pathogens transported by sperm". Q Rev Biol 68 (3): 335–86. doi:10.1086/418170. PMID 8210311.
66. "Canine False Pregnancy and Female Reproduction". Mar Vista Animal Medical Center. 2008-02-02. Retrieved 2008-10-05.
67. Studies that found women are more attractive to men when fertile: S.C. Roberts, J. Havlicek, J. Flegr, M. Hruskova, A.C. Little, B.C. Jones, D.I. Perrett and M. Petrie, M. (August 2004). "Female facial attractiveness increases during the fertile phase of the menstrual cycle". Proc.R.Soc.Lond.B (Suppl.) 271: S270–2. doi:10.1098/rsbl.2004.0174.
Geoffrey Miller, Joshua M. Tybur and Brent D. Jordan (June 2007). "Ovulatory cycle effects on tip earnings by lap dancers: economic evidence for human estrus?" (PDF). Evolution and Human Behavior 28 (6): 375. doi:10.1016/j.evolhumbehav.2007.06.002. Retrieved 2008-01-21.
68. Study that found male sexual behavior is not affected by female fertility: Susan B. Bullivant, Sarah A. Sellergren, Kathleen Stern, et al. (February 2004). "Women's sexual experience during the menstrual cycle: identification of the sexual phase by noninvasive measurement of luteinizing hormone". Journal of Sex Research 41 (1): 82–93. PMID 15216427.
69. Lanting, Frans; Waal, F. B. M. de (1997). Bonobo: the forgotten ape. Berkeley: University of California Press. pp. 107. ISBN 0-520-20535-9. Retrieved 2007-09-05.
70. Stanford, Craig B. (March-April 2000). "The Brutal Ape vs. the Sexy Ape? The Make-Love-Not-War Ape". American Scientist 88 (2): 110. doi:10.1511/2000.2.110.
All text is available under GNU Free Documentation License. Copyrights Disclaimer